Scanning electron microscopy for biological imaging
Scanning electron microscopy (SEM) is a versatile technology that enables the imaging and analysis of various sample sizes and types across different biological disciplines. Thermo Scientific SEMs provide excellent imaging quality, even for challenging materials or when observing minute details. These SEMs support a wide range of techniques and allow for dynamic experimentation, making them suitable for diverse biological research needs.
Large area 2D imaging of biological samples
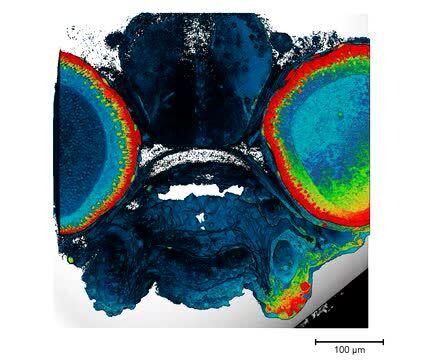
Two-dimensional imaging using scanning electron microscopy (SEM) is a powerful technique that allows for detailed visualization and analysis of the surface topography of various specimens. SEM utilizes a focused beam of electrons to scan the specimen's surface, generating high-resolution images. This method is particularly useful for studying the microstructure of biological specimens, providing valuable insights into their composition and organization at the cellular and subcellular levels.
Environmental SEM: surface topography under environmental conditions
Scanning electron microscopes, such as the Thermo Scientific Quattro ESEM, can image the surface topography of biological specimens while maintaining environmental conditions. This means that biological specimens can be analyzed in their natural hydrated state, even under extended pressures and with the presence of water vapor. As a result, fine surface details can be observed without any artifacts that may be caused by a dehydration process.
Environmental scanning electron microscopy (ESEM) is a special technique used to image biological samples in their natural, hydrated state. Unlike traditional SEM, ESEM can examine samples without dehydration. Special environmental chambers maintain a controlled, moist atmosphere. ESEM is valuable for the study of dynamic biological processes, such as the behavior of living cells, tissues, or microorganisms, and provides high-resolution images without extensive sample preparation.

Near-native imaging with cryo-SEM
The freezing of biological samples provides an alternative to chemical fixation for sample preparation. By vitrifying samples, the ultrastructure of cells and tissues can be preserved. This method allows the samples to remain fully hydrated, eliminating the need for fixatives or resin embedding.
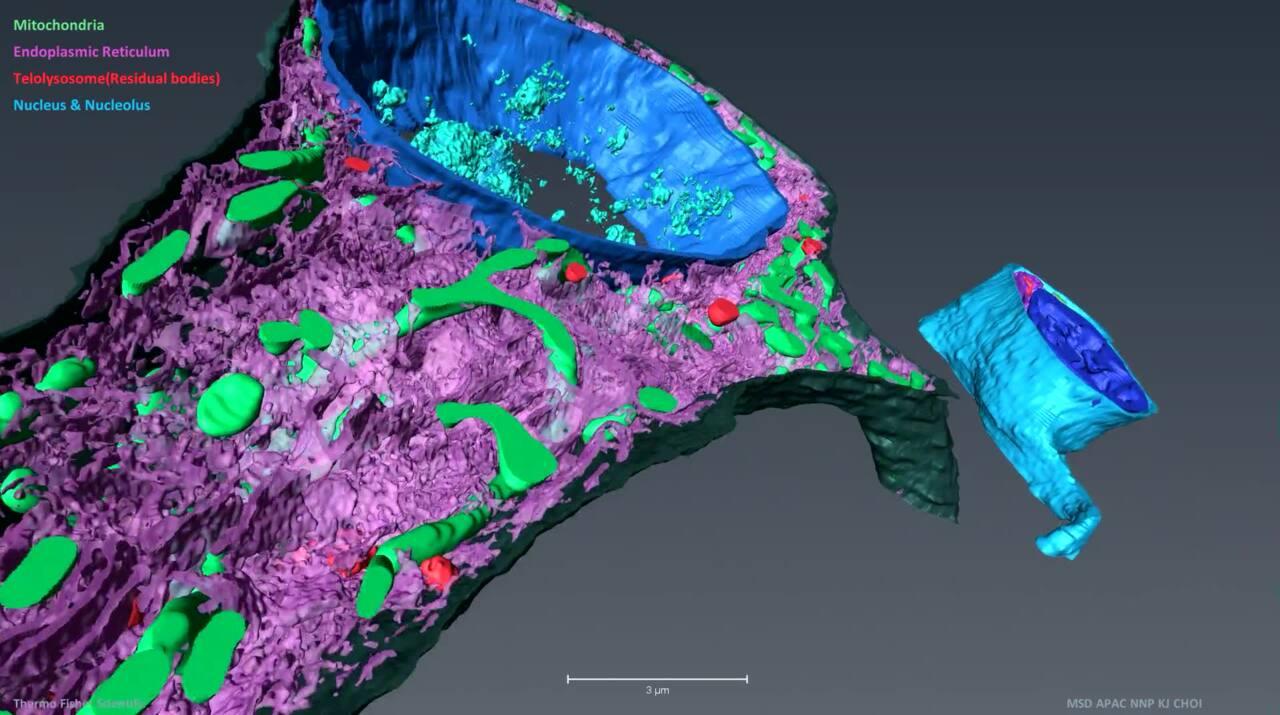
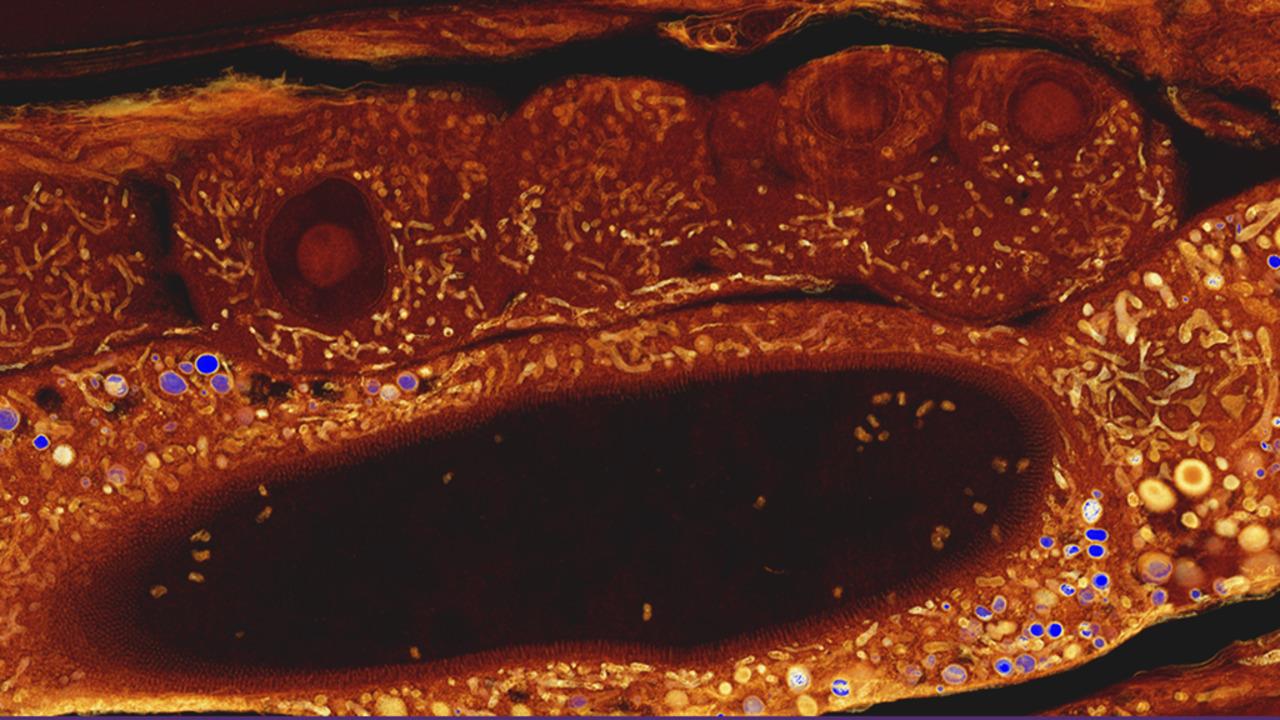
Volume electron microscopy: 3D biological imaging
Understanding the intricate 3D architecture of cells and tissues within their natural context is vital for establishing the correlation between structure and function in biological systems. In recent years, significant advancements have been made in SEM-based techniques for reconstructing large tissue volumes in 3D. One such technique is serial block-face SEM (SBF-SEM), which combines in situ sectioning and imaging of plastic-embedded tissue blocks within the SEM vacuum chamber. This automated method allows for the reconstruction of large tissue volumes in 3D. Another versatile microscopy method is array tomography, which enables the exploration of cell and tissue architectures in three dimensions. It offers the ability to image large tissue volumes with exceptional structural and molecular detail. Array tomography, with its expansive imaging field, is particularly well-suited for tissue histology.
Scanning transmission electron microscopy with SEM for life sciences research
Scanning transmission electron microscopy (STEM) mode in SEM enables imaging of ultrathin resin embedded biological sections resulting in TEM-like images. While STEM systems have been available on TEM instruments for some time, they have not been commonly used for ultrastructural examination in cell biology and pathology. Yet now, scientists can achieve near-TEM quality images when studying thin, soft tissue samples using a scanning electron microscope (SEM). Pairing a scanning transmission electron microscopy (STEM) detector in a SEM, users can obtain high-resolution images of soft tissues at low energy levels to get the contrast they need while preserving the structure of their samples.
Correlative light and electron microscopy
Correlative light and electron microscopy is a combination of fluorescence microscopy with high-resolution electron microcopy. Thermo Scientific Maps Software allows the combination and correlation of data from multiple microscopes, light, SEM, FIB-SEM and TEM instruments for high-throughput data collection. Correlative light and electron microscopy (CLEM) workflow with Maps Software can combine microscopy (LM) images, with its comparably large field of view and fluorescence labels with electron microscopy (EM) data to obtain subcellular ultrastructural information. Additionally, Maps Software can automate the acquisition of a series of tiled images to create an overview of the sample. The resulting images can be stitched together automatically to form a single overview image.
Scanning electron microscopy resources
Scanning electron microscopy application notes
Scanning electron microscopy webinars
For Research Use Only. Not for use in diagnostic procedures.