Figure 1. Peptide recovery of native peptides at various loads using the Pierce Peptide Desalting Spin Columns. Digested HeLa extracts (5–5,000 μg) were loaded onto Pierce Peptide Desalting Spin Columns and processed according to the protocol. Total recovered desalted peptides were estimated using the Pierce Quantitative Colorimetric Peptide Assay.
After isolation of peptides, salts and buffers can be removed using reversed phase (RP) resins, of which the C18 matrix is the most ideal for the capture of hydrophobic peptides. The peptides bind to reverse-phase columns in high-aqueous mobile phase, salts and buffers are washed off, and the peptides are eluted using a high-organic mobile phase. However, hydrophilic peptides, including phosphopeptides, may bind to C18 resins poorly, so graphite spin columns are recommended for better peptide recovery. However, C18 and graphite resins do not efficiently remove detergents, which can contaminate instruments and interfere with column binding, elution and ionization. The Thermo Scientific Detergent Removal products efficiently bind to and remove high concentrations of a wide variety of detergents that are commonly used during sample processing.
- Comprehensive—resins and kits for multiple peptide clean-up strategies
- Optimized—all products are designed to maximize peptide yield and LC-MS compatibility
- Convenient—easy-to-use spin tip and columns enable more rapid clean-up of peptides
- Flexible—devices can be used with either cultured mammalian cells or tissues
- Validated—all products have been fully tested and processed samples have been analyzed using Thermo Scientific Mass Spectrometers
Choose the right peptide clean up products for your application
 |  |  |  |  | |
|---|---|---|---|---|---|
| Pierce Peptide Desalting columns | Pierce C18 Tips | Pierce C18 Spin Columns | Pierce C18 Spin Tips | Graphite Spin Columns | |
| Primary application | High capacity peptide desalting using microcentrifuge | Peptide desalting using micropipette | Peptide desalting using microcentrifuge | Stage tip peptide desalting using microcentrifuge | Phosphopeptide desalting using microcentrifuge |
| Format | Spin column | Tip | Spin column | Spin tip | Spin column |
| Maximum binding capacity | 5 mg | 10 µg or 80 µg | 30 µg | 10 µg | 100 µg |
| Maximum loading volume | 300 µL | 10 µL or 100 µL | 150 µL | 20 µL | 500 µL |
| Processing time | 15 min | 5 min | 25 min | 5-10 min | 10 min |
| Order now | Order now | Order now | Order now | Order now |
Featured data


Figure 2. Peptide recovery of native and TMT-labeled peptides. Pierce Peptide Desalting Spin Columns provide excellent binding and recovery characteristics for native and TMT-labeled peptide samples in preparation for MS. The columns effectively remove excess unreactive TMT label along with contaminating salts. Digested HeLa extract (500 µg) was labeled with TMT isobaric tags and loaded onto each column and processed according to the protocol. Total desalted peptide recovered was estimated using the Pierce Quantitative Colorimetric Peptide Assay.
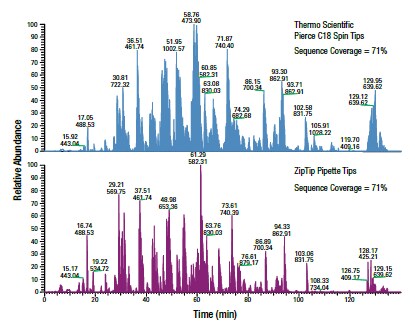
Figure 3. Thermo Scientific Pierce C18 Spin Tips outperform other popular C18 tips. BSA tryptic digests were analyzed on an LTQ Orbitrap XL ETD Mass Spectrometer after processing 10 μg aliquots of the same digest with Pierce C18 Spin Tips or ZipTip™ Pipette Tips (10µL tips with 0.6 µL C18 resin). Base peak chromatograms of the peptide elution were extracted from the data sets to evaluate sample complexity and chromatographic resolution. MS results were analyzed using the SEQUEST search engine and the SwissProt database to determine protein sequence coverage.

Figure 1. Peptide recovery of native peptides at various loads using the Pierce Peptide Desalting Spin Columns. Digested HeLa extracts (5–5,000 μg) were loaded onto Pierce Peptide Desalting Spin Columns and processed according to the protocol. Total recovered desalted peptides were estimated using the Pierce Quantitative Colorimetric Peptide Assay.

Figure 2. Peptide recovery of native and TMT-labeled peptides. Pierce Peptide Desalting Spin Columns provide excellent binding and recovery characteristics for native and TMT-labeled peptide samples in preparation for MS. The columns effectively remove excess unreactive TMT label along with contaminating salts. Digested HeLa extract (500 µg) was labeled with TMT isobaric tags and loaded onto each column and processed according to the protocol. Total desalted peptide recovered was estimated using the Pierce Quantitative Colorimetric Peptide Assay.

Figure 3. Thermo Scientific Pierce C18 Spin Tips outperform other popular C18 tips. BSA tryptic digests were analyzed on an LTQ Orbitrap XL ETD Mass Spectrometer after processing 10 μg aliquots of the same digest with Pierce C18 Spin Tips or ZipTip™ Pipette Tips (10µL tips with 0.6 µL C18 resin). Base peak chromatograms of the peptide elution were extracted from the data sets to evaluate sample complexity and chromatographic resolution. MS results were analyzed using the SEQUEST search engine and the SwissProt database to determine protein sequence coverage.
Video of C18 spin tips in action
Other products for peptide clean up and detergent removal for mass spec
Resources
For Research Use Only. Not for use in diagnostic procedures.