Introduction
Analysis of biological samples in the most physiologically relevant state with minimal sample preparation and manipulation is a key objective in many cell biology workflows. Normally, whole blood samples require time-consuming enrichment and manipulation in preparation for analysis on conventional hydrodynamic focusing flow cytometers. These manipulations can result in loss of rare cell types and undesirable phenotypic changes [1]. Acoustic focusing cytometry, introduced with the Attune Flow Cytometer, allows high sample collection rates without any loss in data resolution, thus eliminating the need for pre-acquisition enrichment and manipulation and helping to enable detection of rare events in a timely manner. In human whole blood, red blood cells outnumber white blood cells ~1,000-fold. This creates two hurdles in attempting to analyze whole blood samples without manipulation: 1) collection of a sufficient number of white blood cell events for statistically meaningful data, and 2) distinguishing white blood cells from red blood cells given the high probability of coincident red blood cell events. This application note outlines a strategy for a no-wash, no- lyse approach to a functional assay using Invitrogen pHrodo BioParticles Conjugates and the Attune NxT Flow Cytometer to assess phagocyte function in human whole blood.
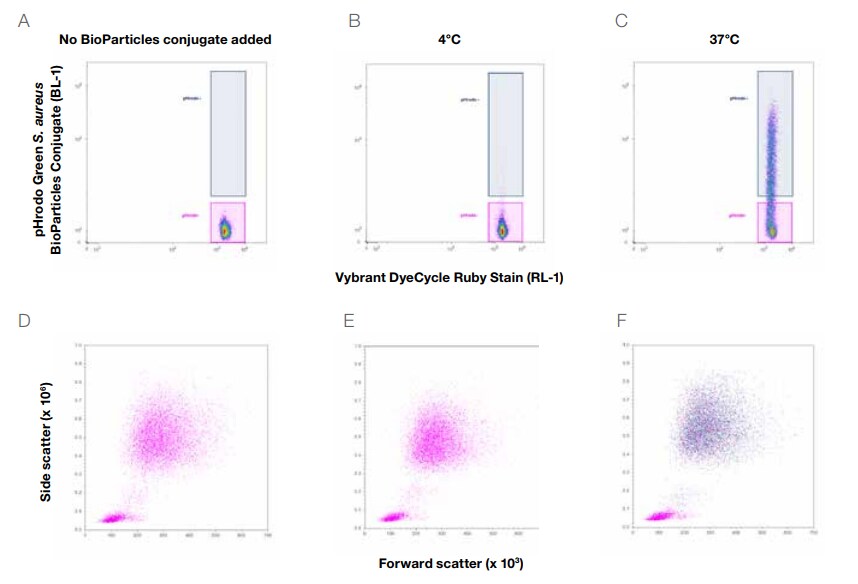
Phagocytic cells are a key component of the innate immune system, serving as a first line of defense against invading pathogens. Neutrophils are the most abundant white blood cells in humans and are often the first cell types recruited to the site of infection where they phagocytose and kill invading bacteria [2]. The significance of neutrophils as a first line of defense against infection is highlighted by certain diseases that result in a reduction of total neutrophil numbers or function, resulting in susceptibility to bacterial infection. There are additional phagocytic cells in blood, including monocytes and dendritic cells. Monocytes mature into cell types such as macrophages or inflammatory dendritic cells upon receiving various stimuli [3]. Dendritic cells are specialized antigen-presenting cells that bridge innate and adaptive immunity. Dendritic cells acquire bacterial antigens through phagocytosis and present these antigens to T cells of the adaptive immune system, which are critical players in mediating protection against certain pathogens [4]. Phagocytosis can be detected using pHrodo BioParticles Conjugates—pH-sensitive reagents that fluoresce upon their ingestion into acidic phagosomes. Characterizing the functional capacity of these phagocytic cell types in a whole blood no-wash, no-lyse assay can save time and reduce the potential artifacts that are introduced in alternative protocols that require red blood cell lysis and multiple purification and wash steps.