Simplify characterization of monoclonal antibodies and other therapeutic proteins
Our advanced instrumentation and analytical workflows can help simplify the challenge of biopharmaceutical analysis. Discover our state-of-the-art analytical technologies for the entire range of protein characterization from post-translational modifications, three-dimensional structures and protein aggregation.
Featured categories
Get faster, more reproducible protein digestion, robust peptide chromatography, and mass spectrometry.
Workflows for intact glycoform, N-glycan, O-glycan, oligosaccharide, monosaccharide, and sialic acid analysis.
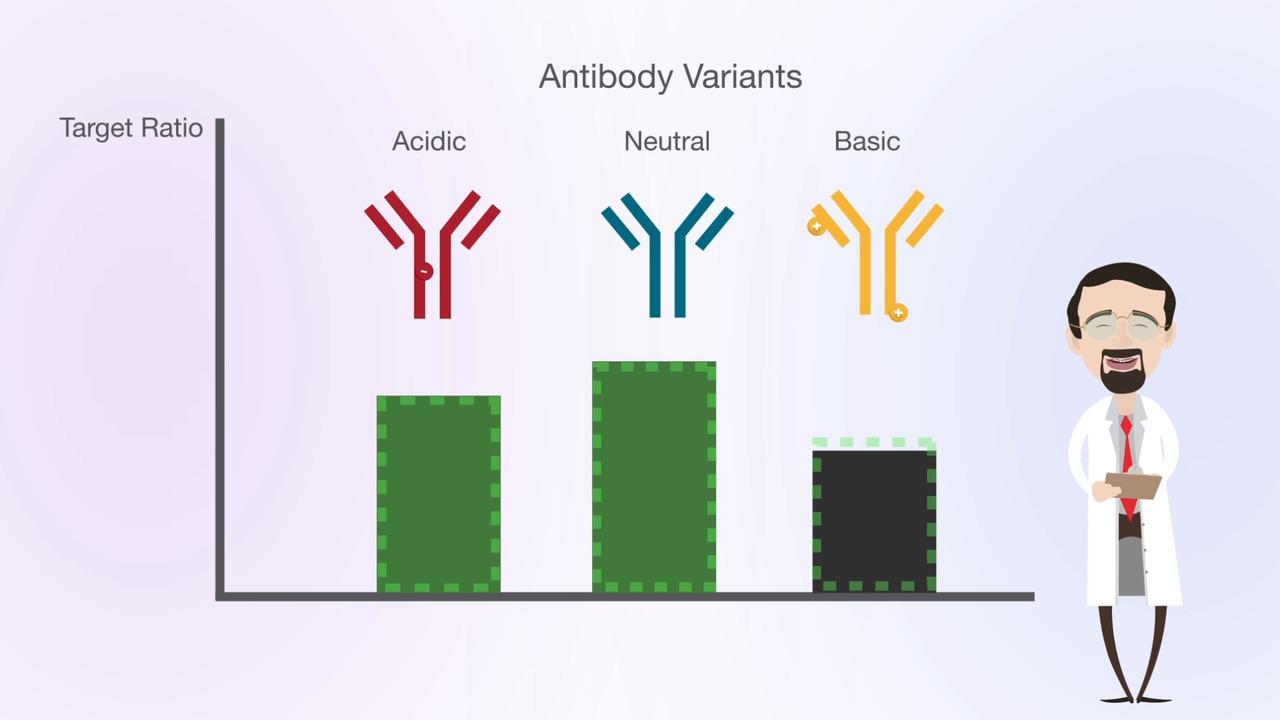
Utilize these workflow solutions for protein intact mass analysis, charge variant profiling, and protein aggregate analysis of biopharmaceuticals.
Gain a deeper understanding higher order structure, including protein conformation, conformation dynamics and protein-protein interactions.
Advanced chromatography and mass spectometry to easily determine drug-antibody ratios (DAR) of drug-conjugates.
Identify and quantify host cell protein related impurities in drug products.
Separate therapeutic and diagnostic oligonucleotides and their derivatives.
Proven workflows for comparative structural protein analyses for biosimilar development.
Proven workflows for comparative structural protein analyses for biosimilar development.
Featured videos
Tomorrow's Quantitation Compendium
Developing robust quantitation assays is possible with both Triple Quadrupole and Orbitrap-based High- resolution MS instruments. Both technologies enable confident quantitation in a variety of sample types.
LC-MS webinar series
Expand your application knowledge
Our LC-MS on-demand webinar series expands your application knowledge for pharma, biopharma, environmental, omics, food, forensics and clinical research and more. Learn how the latest high-performance mass spectrometry solutions can enable your laboratory to solve your most pressing analytical challenges, regardless of sample type.