Beaded agarose media for Immunoprecipitation (IP) historically has several challenges that are difficult to overcome, leading to commonly held beliefs about IP that are not always true. The evolution of the IP technique to utilize magnetic beads has helped the scientific community overcome specific myths about IP, thus providing support for scientists who need help optimizing their IP experiments. Read more below for immunoprecipitation tips.
Immunoprecipitation tip #1: Reduce background in by using magnetic beads
Where does background come from? Background in an immunoprecipitation experiment is an additional, non-specific signal that does not coincide with the weight of your antigen or protein of interest. The background is primarily caused by two sources: 1) Not clearly separating the supernatant from the pellet/resin or beads and 2) The pores from the agarose or Sepharose resin. When using the slurry method with Sepharose or agarose resin, there is no clear pellet. Thus, researchers may end up accidentally removing too much slurry (and precious sample) or not removing enough and leaving the sample with unwanted proteins that give rise to background. Porous Sepharose/agarose resin traps liquid and, along with it, unwanted proteins. Even with several washes, it may still be difficult to remove interfering proteins, leading to high background.
How to reduce background in immunoprecipitation
Dynabeads magnetic beads are magnetized, allowing them to be easily pulled to the side of the sample tube when used with a magnet or magnetic rack. This feature makes it easy to remove buffer/supernatant without any disruption or loss of sample.
A key feature of Dynabeads magnetic beads is their well-defined surface free of pores and holes. All binding occurs on the outer surface thus preventing any unwanted proteins from getting trapped inside.
To further reduce background in immunoprecipitation, it is recommended to thoroughly wash the beads containing the protein of interest to remove any unwanted proteins or protein fragments.
Figure 1. Dynabeads Protein A magnetic beads vs alternative suppliers for Imunoprecipitation. Western blot was conducted to compare protocol times and background using 1) Sepharose spin column, 2) Sepharose slurry, 3) agarose slurry and spin column, 4) resin spin column, and 5) Dynabeads Protein A magnetic beads with n=2 for each method. Dynabeads Protein A magnetic beads IP has low background and saves time by at least 14 minutes. Dynabeads Protein G IP kit is also available.
Video: Busting the myth that background is unavoidable
Immunoprecipitation tip #2: Avoid background without pre-clearing lysates for IP
There is an IP myth that pre-clearing lysates during Immunoprecipitation is necessary for high quality results. However, this assumes that other proteins, which are not the target protein of interest, could bind non-specifically to the solid phase. To address this, a pre-clearing step is done where the solid phase (without the antibody) is added to the sample for a period of time and then removed to potentially remove unwanted, non-specific proteins and background. However, performing the pre-clearing step for IP requires the use of double the amount of solid phase and increases the overall time required for the IP protocol.
With Dynabeads magnetic beads coupled to a specific antibody for your protein of interest, pre-clearing is not necessary, because it does not recognize non-specific proteins. This approach not only saves half of the solid phase used, but also reduces the protocol time.

Figure 2. Dynabeads Protein G magnetic beads without pre-clearing vs Sepharose Protein G beads with pre-clearing. Western blot was conducted to compare background levels between two methods 1) Sepharose Protein G beads with pre-clearing and 2) Dynabeads Protein G magnetic beads without pre-clearing with a sample size n = 8 for each method. Results showed that IP using Dynabeads Protein G magnetic beads consistently precipitates more of the protein of interest. Dynabeads Protein A magnetic beads are also available.
Video: Busting the myth that pre-clearing is necessary
Immunoprecipitation tip #3: Balance binding capacity, yield, and specificity
Some researchers believe that higher binding capacity is better for immunoprecipitations. This IP myth is based on the antigen-binding capacity of agarose and Sepharose beads. Agarose and Sepharose beads have outer and inner surfaces, which is a large total area where proteins can bind, leading to high capacity. This high capacity means these beads and resins can also bind non-specifically to other proteins present in the sample. Researchers need to strike a balance between capacity, yield, specificity, and cost when choosing IP techniques.
Magnetic beads where the outer surface binds to antibodies that recognize your protein of interest can be used. Since only 1–10 µg of antibody is required for Immunoprecipitation outside of using a column-based IP method, higher capacity reagents are unnecessary and cost-ineffective. Only use the exact amount needed for an experiment—this saves your lab antibodies, reagents, and IP beads.
Video: Busting the myth that high-capacity solid phase is crucial
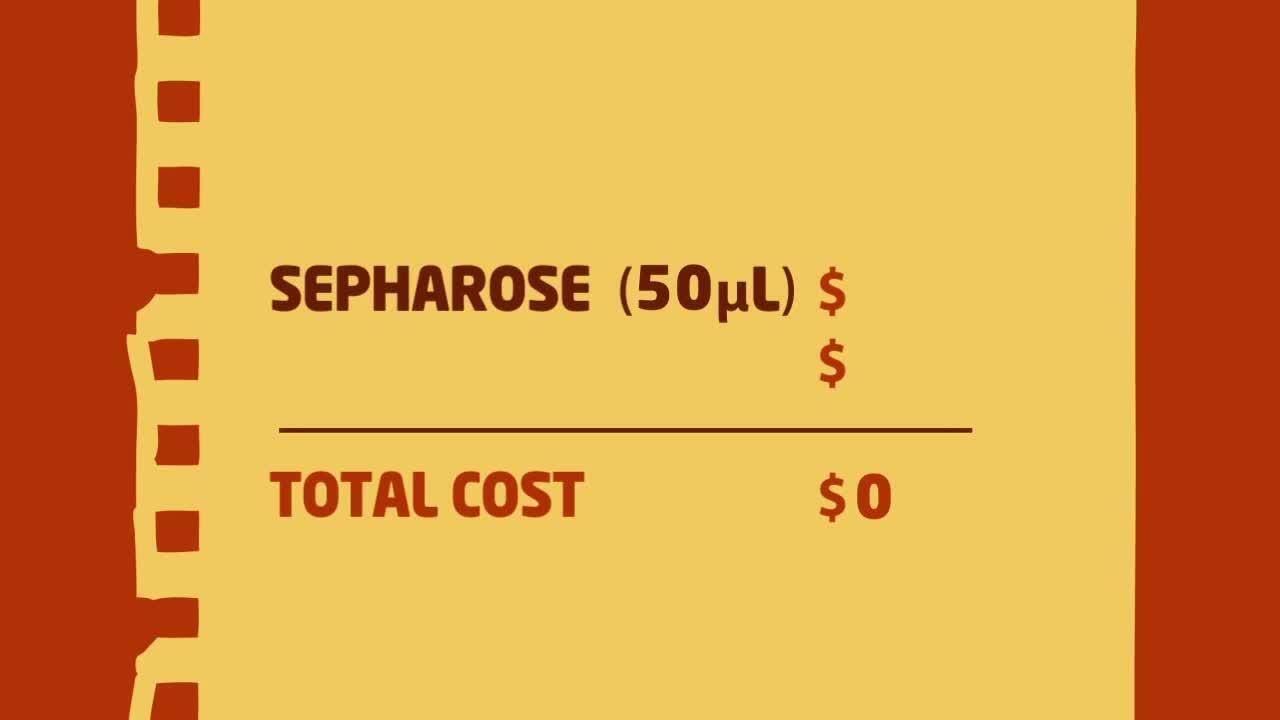
Immunoprecipitation tip #4: Calculate IP cost savings
Researchers may think Dynabeads magnetic beads are expensive while agarose/Sepharose slurries are more cost-effective. However, the price per sample for IP is comparable—if not lower—when you consider excluding the pre-clearing step for magnetic beads.
When calculating the cost of performing IP, researchers may look at the cost of the solid phase relative to the binding capacity of a fixed volume of that solid phase. However, as we learned in Tip #3, the high capacity of the solid phase is not crucial to the IP experiment and can be redundant, resulting in the use of larger amounts of precious, expensive antibody.
The appropriate way to calculate costs of performing IPs is to look at the minimum amounts of beads and antibody needed for one IP. Since agarose/Sepharose slurries usually require pre-clearing, the cost for those beads must double. Because Dynabeads magnetic beads have less non-specific binding and do not require pre-clearing, using Dynabeads magnetic beads for your Immunoprecipitation experiment is more cost-effective than using agarose/Sepharose beads. View the video below to see the exact price amounts.
Video: Busting the myth that quality magnetic beads are not cost-effective
Video: Compare immunoprecipitation methods
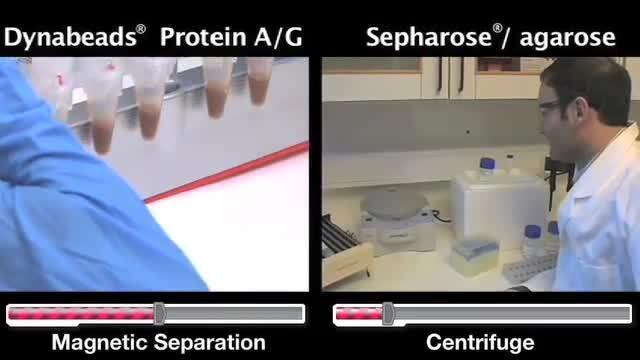
Watch this video compare two IP methods: Dynabeads magnetic beads and Sepharose/agarose slurries.
Video: From sepharose/agarose slurries to magnetic beads for immunoprecipitation
Watch this video demonstrate the shift in the scientific community from the use of Sepharose/agarose slurries to Dynabeads magnetic beads for Immunoprecipitation, as well as overviews of both methods.
Immunoprecipitation related content
Literature
For Research Use Only. Not for use in diagnostic procedures.