Biopharmaceutical characterization is required throughout all stages of drug development and manufacturing. During drug development, liquid chromatography–mass spectrometry (LC-MS) approaches are used to structurally characterize monoclonal antibodies (mAbs) produced using cell lines. The main objective is to determine monoclonal cell populations producing the desired mAb with a high degree of fidelity and activity for a specific antigen. The analysis of intact biologics is continued throughout manufacturing to assess purity and heterogeneity characteristics for lot release purposes.
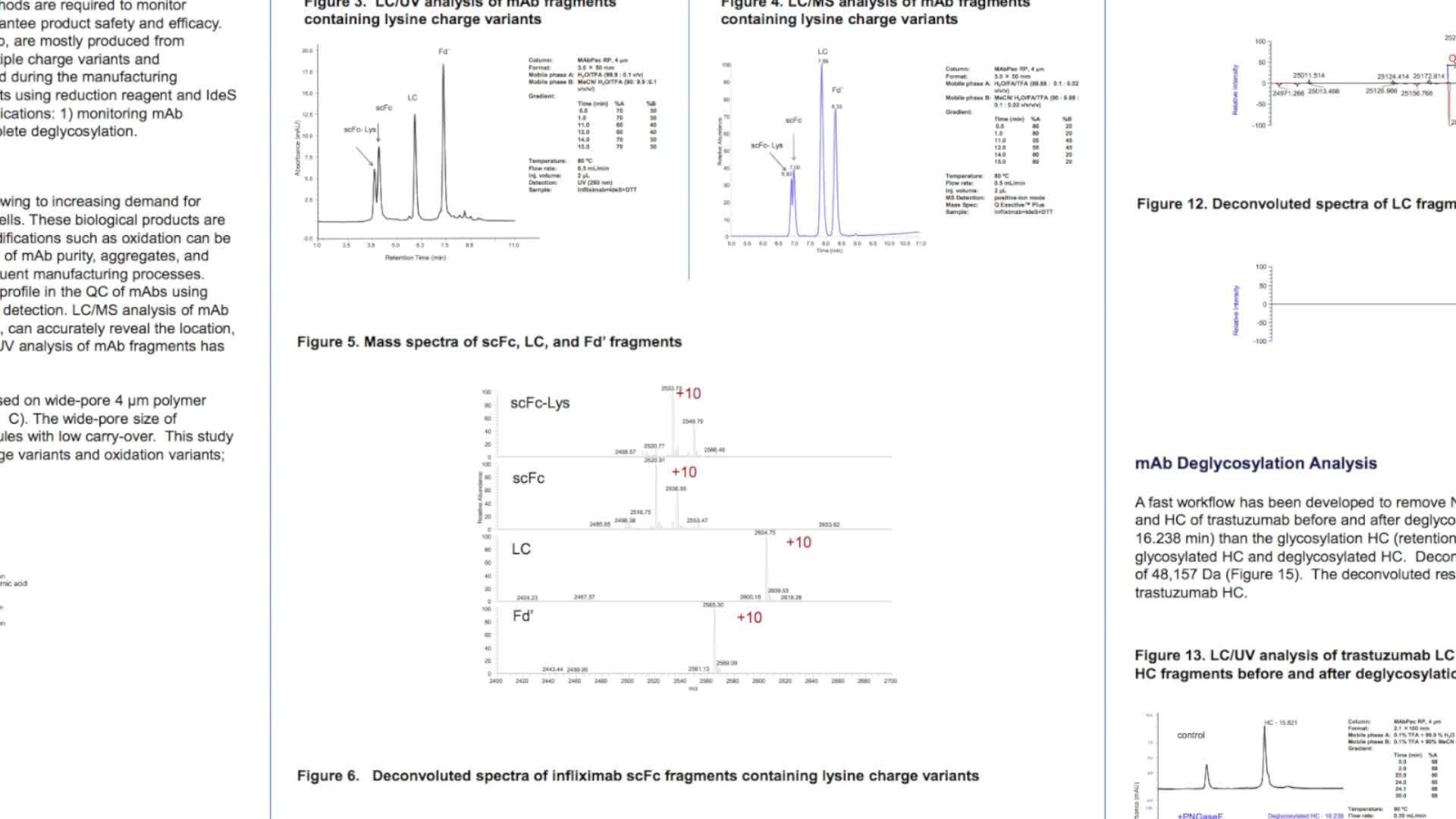
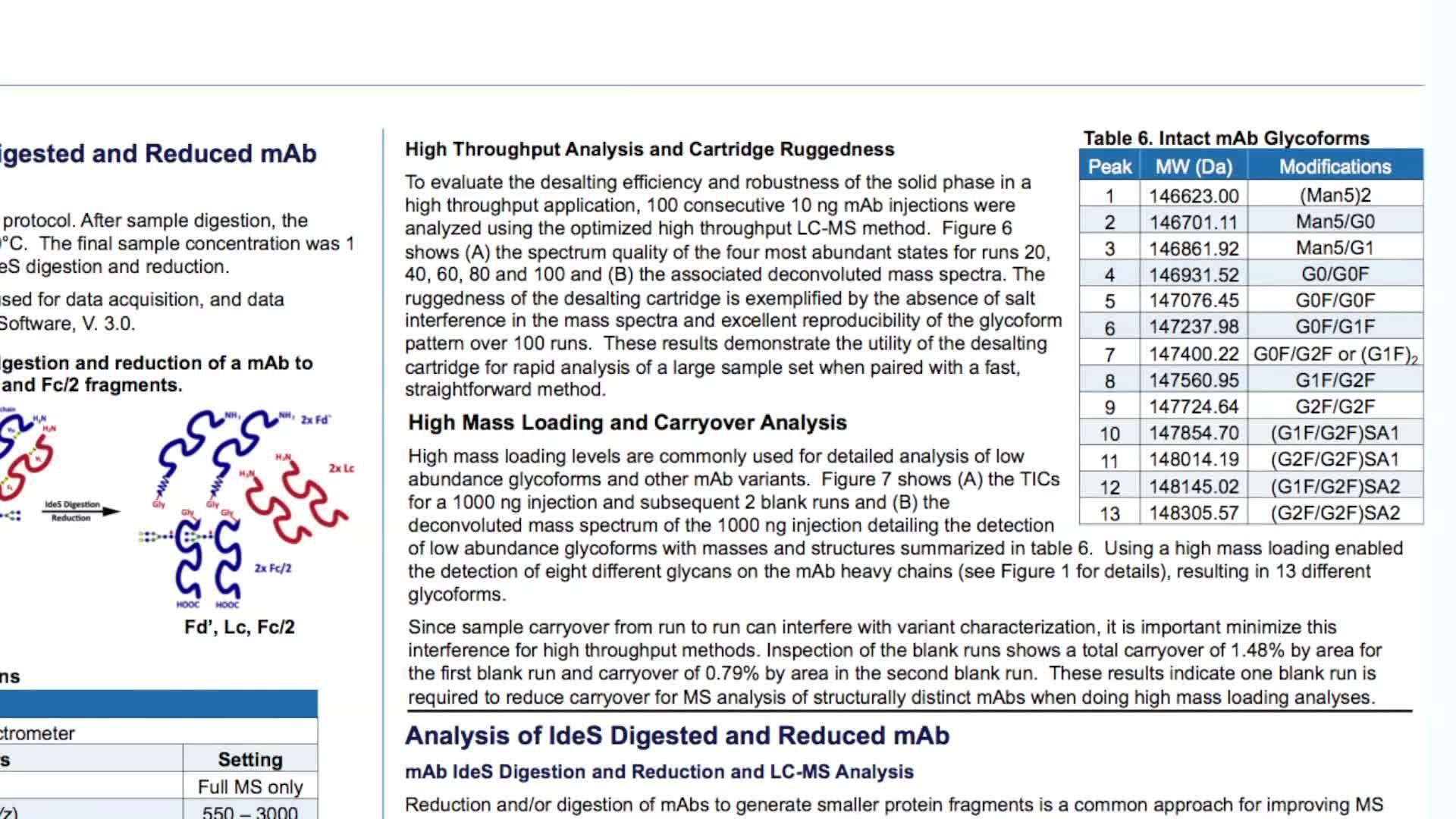
Multiple analytical approaches can be utilized to assess biotherapeutic molecules in their intact state. Information on accurate protein mass and heterogeneity can be provided by MS analysis following a short desalting step and is important for quantification of the various glycoforms and protein variant analysis, important considerations for therapeutic drug formulation and storage. Biotherapeutic drugs can be analysed at their subunit level following reduction and/or digestion. Analysis of therapeutic antibody subunits facilitates exact molecular weight analysis of their heavy and light chains and further top down analysis can provide sequence confirmation.
Protein analysis in native or native-like conditions decreases charge state value resulting in mAb detection at higher m/z ranges with more spatial resolution. For example, native mass spectrometry conditions also allow the preservation of structurally-critical non-covalent bonds which may be necessary to the analysis of cysteine-linked antibody-drug conjugates (ADC)