Product Details
A14010
Host/Isotype
Class
Type
Immunogen
Conjugate
Form
Storage conditions
Shipping conditions
RRID
Target Information
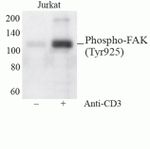
Focal Adhesion Kinase (FAK) is a 125 kDa non-receptor protein tyrosine kinase that acts as a substrate for Src and is a key element of integrin signaling. FAK plays an important role in cell spreading, differentiation, migration, cell death, and acceleration of the G1 to S phase transition of the cell cycle. FAK has a central catalytic domain and a C-terminal tail that localizes it to focal adhesions, which are sites where cells attach to the extracellular matrix via surface integrin receptors. Increased FAK tyrosine phosphorylation occurs upon integrin engagement with fibronectin. Adhesion of murine NIH3T3 fibroblasts to fibronectin promotes association of the Grb2 adapter protein and c-Src PTK with FAK in vivo, and also results in activation of the ERK2 MAP kinase. In v-Src-transformed NIH3T3, the association of v-Src, Grb2, and Sos with FAK is independent of cell adhesion to fibronectin. In vitro the Grb2 SH2 domain binds directly to tyrosine-phosphorylated FAK, and the binding site has been identified as Tyr925 by site directed mutagenesis. Several transcript variants encoding different isoforms have been found for the FAK gene, but the full-length natures of only three of them have been determined.
For Research Use Only. Not for use in diagnostic procedures. Not for resale without express authorization.
References (0)

Performance Guarantee
If an Invitrogen™ antibody doesn't perform as described on our website or datasheet,we'll replace the product at no cost to you, or provide you with a credit for a future purchase.*
Learn more
We're here to help
Get expert recommendations for common problems or connect directly with an on staff expert for technical assistance related to applications, equipment and general product use.
Contact tech support