What is microcrystal electron diffraction (MicroED)?
MicroED is a technique that allows for fast, high-resolution structural determination of small molecules and proteins. Atomic details can be extracted from individual nanocrystals (<200 nm in size), even in a heterogeneous mixture. MicroED data is acquired on a cryo-transmission electron microscope (cryo-TEM), using electrons as the incident beam.
Since MicroED is a diffraction technique, samples need to be crystalline, and the crystallization process is the same as for X-ray crystallography. However, much smaller crystals can be used in MicroED because the interaction of the crystal with electrons is much stronger than it is with X-rays. Crystals that are only 100 nm in size can readily be analyzed. This may significantly shorten the sample preparation process and allows for the analysis of crystals that are too small to diffract with other methods. Data collection is completed in only a few minutes, and 3D structures can be determined at atomic resolution.
Sample requirements for MicroED
The unique requirements of MicroED set it apart, not just from other cryo-electron microscopy (cryo-EM) techniques, but from traditional crystallography as well. MicroED necessitates small crystals (<50 µm) but samples can be cut (or milled) from larger crystals using a focused ion beam (FIB), if necessary. (Note that crystals >200 nm have increased secondary scattering, which convolutes the data.)
Sample preparation also varies between small molecule and protein samples. Small molecule crystals are usually dry and can often be analyzed at room temperature. Mechanical grinding can easily be used to reduce the size of large crystals, or the molecules can simply be crystallized spontaneously out of solution using evaporation.
Protein crystals, however, are typically kept in water to retain their hydrated, native states. These samples are subsequently flash-frozen (vitrified) in order to avoid sample damage due to crystalline ice formation. Vitrified samples are sensitive to changes in humidity or to the buffer and may disintegrate at the slightest touch. Therefore, cryo-FIB milling is used to reduce the size of large protein crystals.
Key benefits of MicroED
MicroED fills a gap in structural biology, since X-ray crystallography requires large crystals that can be difficult to obtain whereas synchrotron X-ray free-electron lasers (XFELs) necessitates hundreds, if not thousands, of small crystals for the structural analysis of a single protein or small molecule. Time on a synchrotron is limited. MicroED uniquely offers fast, high-resolution analysis that requires only a few small crystals.
Fast, atomic-resolution 3D structural information
Obtain diffraction data from nanocrystals in minutes
Complete turn-key solution
Acquired data can be processed using established reconstruction packages for X-ray crystallography.
Instant productivity
Nanocrystals as small as 100 nm can readily be analyzed, removing the burden of large crystal growth (as needed in X-ray crystallography). This also reduces the amount of sample material required. Mixtures of different polymorphs and compounds can be analyzed.
2-in-1 solution
MicroED and single particle analysis can be performed on the same cryo-electron microscope. This solution is compatible with new microscopes but can also be retrofitted onto existing cryo-EM instruments.
MicroED use cases
Drug discovery using microcrystal electron diffraction
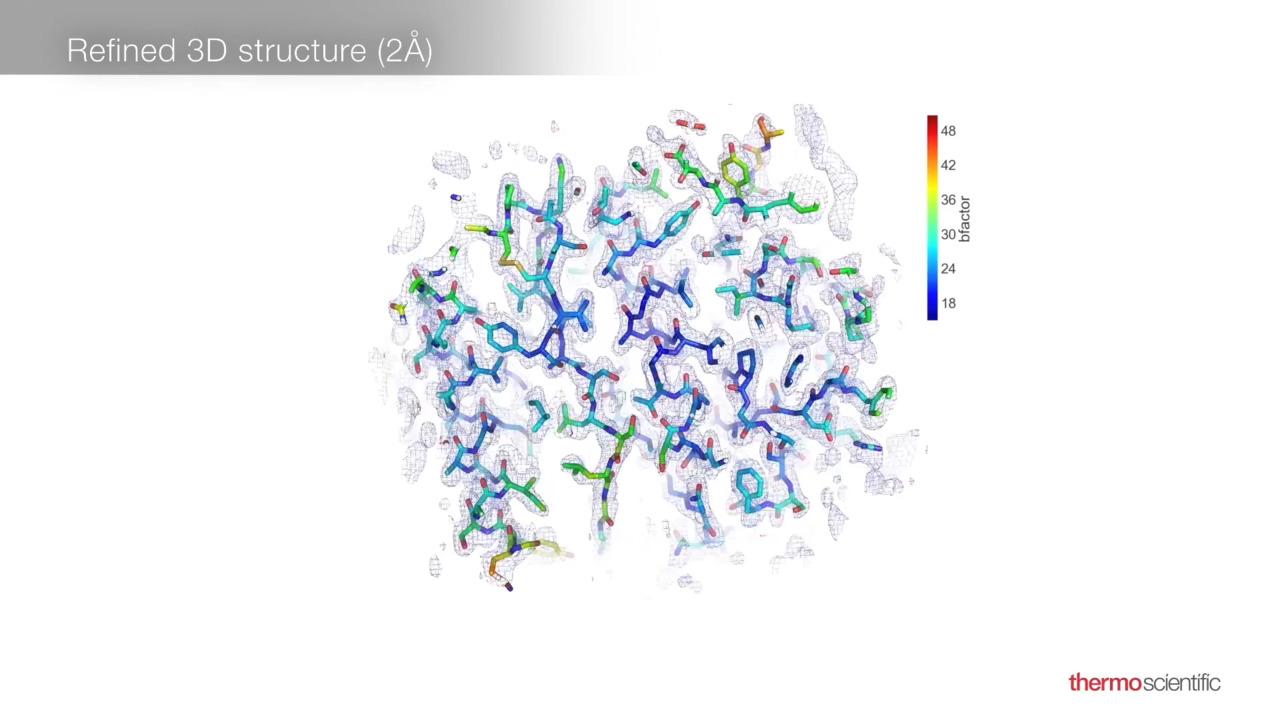
MicroED can be used to determine the structure of protein-drug complexes and small molecules. Critically, MicroED alleviates the need for long and complicated large-crystal growth, a significant benefit in the fast-paced pharmaceutical industry. To highlight some of the key benefits of MicroED, we determined the structure of acetaminophen from a commercial sample. Only ~10-12 grams of the sample were required to obtain the structure shown; the nanocrystal was also extracted from a mixture consisting of filler and other compound constituents. The entire 70-degree range of data was collected in a matter of minutes.
Data processing was performed in the open-source, Diffraction Integration for Advanced Light Sources (DIALS) software, as outlined below. It is important to note that while the full structure could be determined from a dataset that was only 43% complete, additional data from other crystals could be used to further enhance these results.
General outline of MicroED data processing performed with open-source Diffraction Integration for Advanced Light Sources (DIALS) Software.
Microcrystal electron diffraction of organometallics
A recent study from the University of York showcases how 200 kV cryo-TEM can be used for the MicroED analysis of single crystal organometallics. Specifically, single crystal to single crystal (SC-SC) transformations, performed on grid, can be used to generate and characterize otherwise highly reactive organometallic species. Here, nanocrystals composed of a norbornadiene complex were hydrogenated by the addition of H2 gas into a σ-alkane (norbornane) complex. The resulting crystallographic structures were resolved down to 0.95 Å resolution. These results not only showcase the quality of MicroED data that can be generated at 200 kV, but more broadly demonstrate the potential of cryo-EM for on-grid single-crystal chemistry.
Comparison of MicroED data collected from nanocrystals (C) with traditional X-ray diffraction results obtained from larger crystals, which were much more difficult to obtain. Reproduced from the corresponding article by LR Doyle et al under CC BY 3.0.
Meet the scientists using MicroED
“The electron microscopes these days are so powerful that it's really like having a synchrotron beamline in your own laboratory.”
Tamir Gonen, Professor at University of California in Los Angeles
MicroED resources
For Research Use Only. Not for use in diagnostic procedures.