The power of cryo-EM in virology
Viruses are miniature, highly efficient gene delivery machines. These infectious agents come in a wide variety of shapes and sizes, with most having a diameter between ten and a few hundred nanometers. Due to their size, analytical techniques such as electron microscopy provide valuable insights for diagnosis, treatment, and vaccine development.
Transmission electron microscopy is routinely used for fast virus detection and identification in diagnostic settings. Beyond viral diagnoses, electron microscopy is also on the forefront of virology, as it is used in virus structure and pathogenesis studies, especially though near-atomic resolution instruments like cryo-electron microscopes (cryo-EM). Cryo-EM features several techniques that can image virus structure, viral proteins, and virus-antibody immune complexes in 3D. Single particle analysis is a cryo-EM technique capable of determining high-resolution structures of macromolecular complexes such as the "spike" protein of coronaviruses. The cryo-EM technique known as cryo-electron tomography has provided structural information with broader cellular context, such as viral assembly within bacteria.
Preparing for the next major virus
Viruses will doubtlessly continue to evolve and affect our world in devastating ways. Minimizing their impact will rely on the development of treatments and preventative measures driven by highly detailed insight into the nature and structure of viruses. Cryo-EM has been used to study virus morphology for over 20 years, resolving the structure of viruses such as Zika, Ebola, HIV, and coronaviruses. The near-atomic-resolution information provided by cryo-EM is critical for a better understanding of the molecular mechanisms behind antibody-antigen interactions. In fact, cryo-EM structures are increasingly used in protein epitope mapping, defining specific binding sites. These iterative studies aid the understanding of antibody mutations for faster discovery and development of more specific and effective vaccines or antiviral treatments.
Battling viruses at atomic scale with cryo-EM
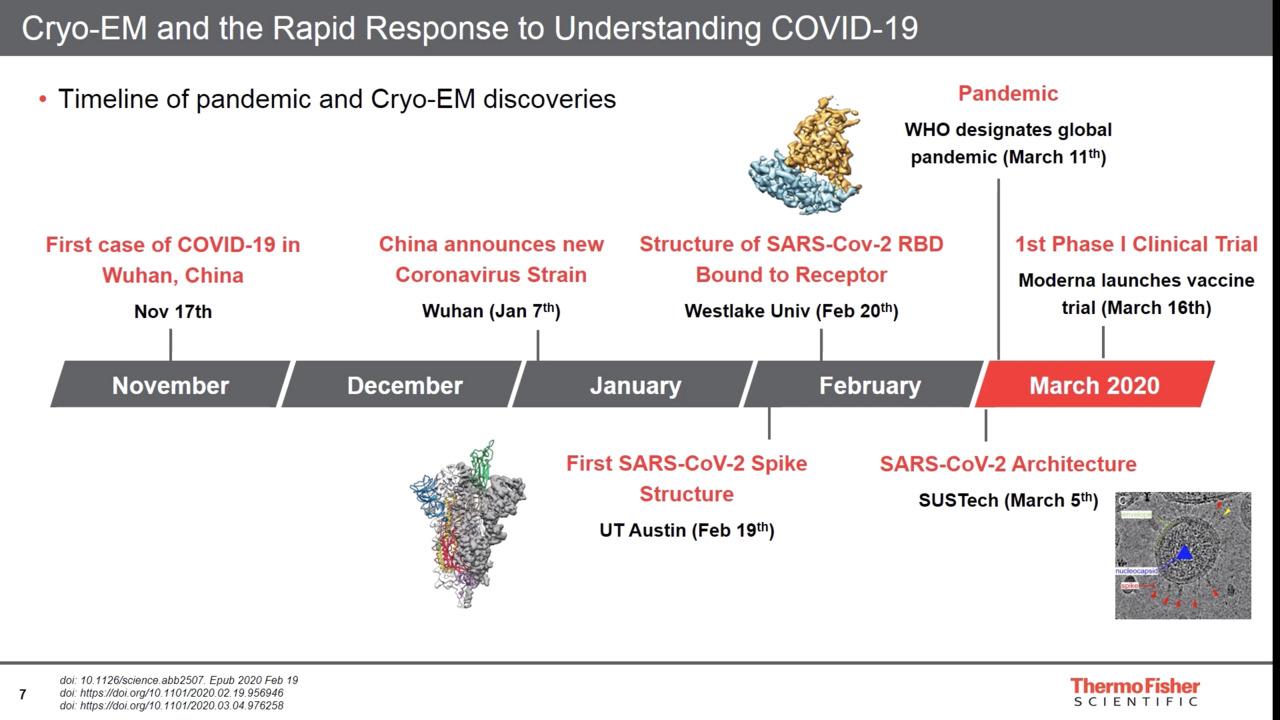
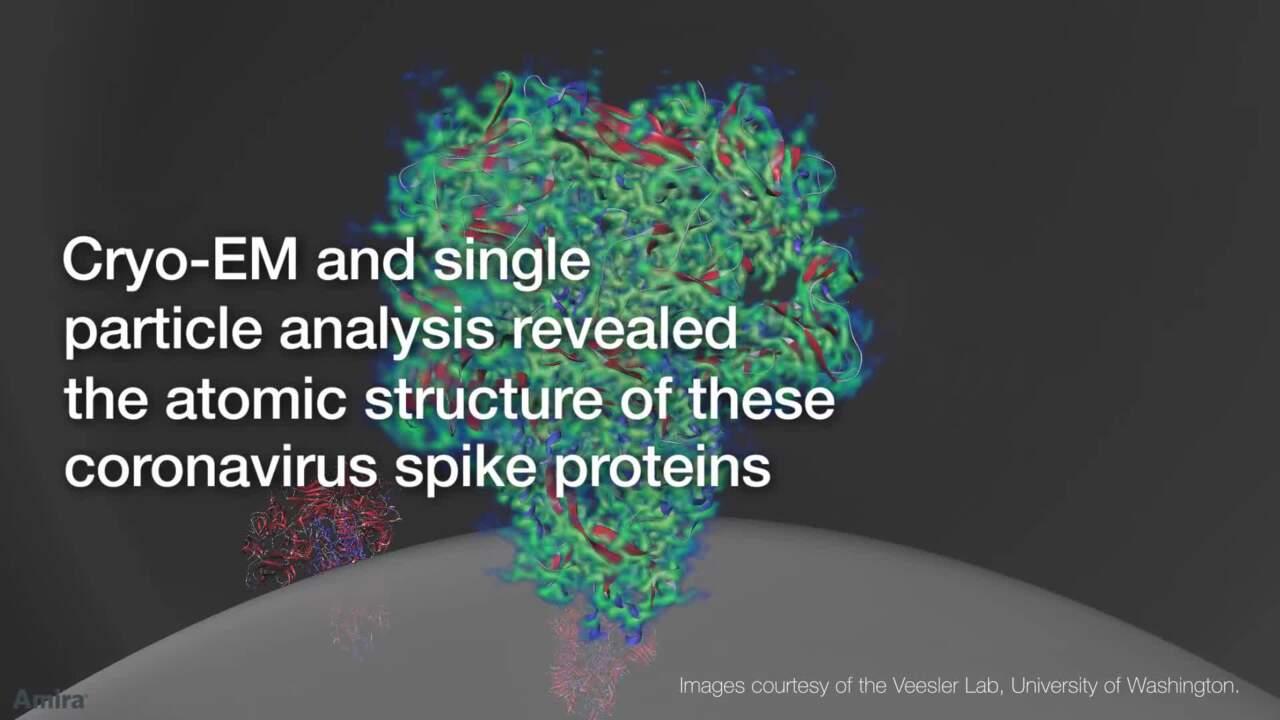
Researchers have used electron microscopy, particularly cryo-EM, to increase our understanding of the SARS-CoV-2 virus. In January 2020, a Thermo Scientific 120kV TEM was first used to directly image the novel coronavirus in human cells at the National Institute for Viral Disease Control and Prevention (NIVDC), part of the China Centers for Disease Control in Beijing. In February, cryo-EM researchers determined the structure of the SARS-CoV-2 spike protein and its cellular receptor during infection using the Thermo Scientific Krios Cryo-TEM. By quickly solving the structure of the coronavirus spike protein, researchers used these results to develop a vaccine that entered phase 1 clinical trials in March 2020. Since then, more and more research groups around the world use cryo-EM to gain a detailed understanding of SARS-CoV-2 and to aid the development of vaccines, antivirals, and neutralizing antibodies.
Cryo-EM in Zika virus research
The Zika virus, while initially transmitted by mosquito or tick, is infamous for passing from mother to unborn child, leaving the infant with a range of developmental and neurological defects. Researchers at Purdue University used cryo-EM to determine the structure of the entire virus particle with a 3.1 Å resolution; this is vital as viruses in this family appear otherwise identical at lower resolutions. These observations helped contribute to two potential vaccines currently in human trials.
Cryo-EM in Ebola virus research
Highly infectious and often fatal, outbreaks of the Ebola virus have imminently devastating consequences. Despite the danger it poses, there is still no effective treatment for this disease. Given the inherent danger of working with this virus, researchers are focused on studying viral components, which promise to accelerate the discovery of treatments and vaccines. For example, researchers at the Okinawa Institute of Science and Technology (OIST) determined the structure of the nucleocapsid-like assembly (which houses the genetic material of the virus) at near-atomic resolution. Understanding this component of the Ebola virus allows researchers to more accurately target the mechanism and spread of this deadly disease.
Cryo-EM in HIV research
As a retrovirus, HIV introduces a copy of its genetic material into the DNA of the host, hijacking the machinery of the cell to produce further copies of itself. Research is focused on preventing the initial binding of the virus to the host cell and the integration of its genetic material into the host DNA. Researchers at Harvard Medical School employed cryo-EM to capture the structure of the fusion protein complex, which is believed to initiate the binding of the virus to the host cell. By fully understanding this structure, effective treatments that inhibit the binding mechanism can be produced.
Cryo-EM in poliovirus research
According to the Center for Disease Control (CDC), polio was once one of the most feared diseases in the United States, causing more than 15,000 cases of paralysis per year in the early 1950s. But in 1955, people across the country rejoiced at the announcement of an approved poliovirus vaccine, and by 1979 the CDC said that because of widespread vaccination, polio had been eliminated from the United States. Yet, poliovirus has remained endemic in countries like Pakistan and Afghanistan, and the United States hasn’t been without transmission over the past 40 years, albeit extremely low. Cryo-EM is being used to understand how poliovirus infects cells and replicates.
Cryo-EM in biosafety level labs
Despite the achievements of structural virology, many viruses have not been studied yet, and questions about their life cycles and interactions with host cells remain unanswered. These studies are essential, as they lay the foundation for interventions against existing and novel diseases.
However, work on pathogens that pose a risk for disease requires a biosafety containment facility unless work is limited to isolated proteins or inactivated samples. High-end cryo-EM equipment is not commonly found in these facilities.
At Thermo Fisher Scientific, our mission is to enable our customers to make the world healthier, cleaner, and safer. We have been building expertise and are developing products and services so that our customers can accelerate their pathogen research using cryo-EM. Our Application Development Laboratory houses our cryo-EM instruments in a biosafety level 2 (BSL-2) environment.
Heat decontamination for cryo-TEM
Empower your lab with the new 60°C heat decontamination for the Thermo Scientific Krios G4 Cryo-Transmission Electron Microscope (cryo-TEM). This accessory enables pathogen research and comprehensive instrument service in higher biosafety-level containment facilities (e.g. BSL3), so you can help make the world cleaner, safer, and healthier.
Our new solution for 60°C heat decontamination allows installation of the Krios G4 Cryo-Transmission Electron Microscope (Cryo-TEM) in higher biosafety-level containment facilities (e.g. BSL-3). Our remote diagnostics and repair capabilities accelerate support for these installations and reduce the need for physical access. We will partner with you to tailor a service contract that supports your expertise and organization.
Thermo Fisher Scientific is dedicated to working with our customers to further leverage cryo-EM’s strength for virus research.
Cryo-EM service solutions tailored for biosafety needs
Excellent support is available for systems in a higher biosafety-level containment facility, including BSL-3, through a comprehensive set of service solutions tailored to the circumstances of your location. Protocols and processes specific to the biosafety level of your lab secure a safe working environment for our engineers and our complete supply chain.
Whenever possible, we will employ our remote diagnostics and repair capabilities, accelerating the time to solution and reducing the need for physical access to the system. On-site support is available and is provided under your authority to ensure the safety of our service personnel. Connected Care services and applications support packages are available to accelerate your research and enhance productivity.
For Research Use Only. Not for use in diagnostic procedures.