Product Details
Z2478MS
Species Reactivity
Host/Isotype
Class
Type
Clone
Immunogen
Conjugate
Form
Concentration
Purification
Storage buffer
Contains
Storage conditions
Shipping conditions
Product Specific Information
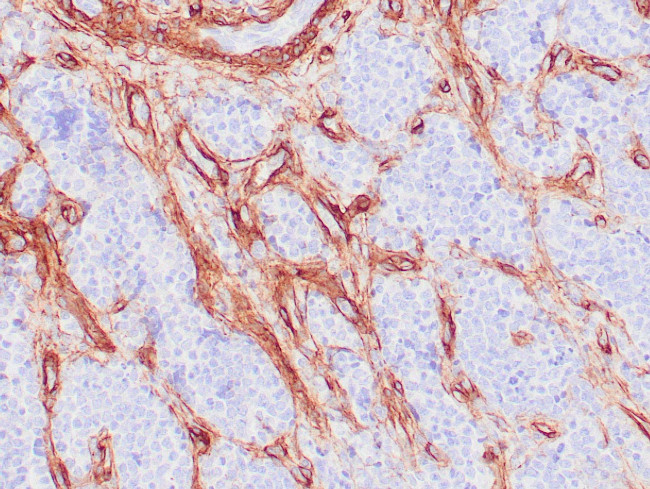
A recommended positive control tissue for this product is Leiomyosarcoma, however positive controls are not limited to this tissue type.
The primary antibody is intended for laboratory professional use in the detection of the corresponding protein in formalin-fixed, paraffin-embedded tissue stained in manual qualitative immunohistochemistry (IHC) testing. This antibody is intended to be used after the primary diagnosis of tumor has been made by conventional histopathology using non-immunological histochemical stains.
Collagen Type IV is a major component of the basement membrane and plays an important role in cell adhesion, migration, differentiation and growth. Collagen Type IV express at the basement membranes in a variety of tissues including kidney, muscle, lymph nodes, lung, tendon and spleen. Collagen Type IV has been shown to be useful in differentiating microinvasive from in situ ductal carcinomas of the breast. Other Collagen Type IV studies include use in pancreatic adenocarcinoma and chronic pancreatitis, nephrosclerosis and other kidney diseases, oral squamous cell carcinoma, laryngeal cancers, ovarian cancers and cervical cancers.
Antibody is used with formalin-fixed and paraffin-embedded sections. Pretreatment of deparaffinized tissue with heat-induced epitope retrieval or enzymatic retrieval is recommended. In general, immunohistochemical (IHC) staining techniques allow for the visualization of antigens via the sequential application of a specific antibody to the antigen (primary antibody), a secondary antibody to the primary antibody (link antibody), an enzyme complex and a chromogenic substrate with interposed washing steps. The enzymatic activation of the chromogen results in a visible reaction product at the antigen site. Results are interpreted using a light microscope and aid in the differential diagnosis of pathophysiological processes, which may or may not be associated with a particular antigen.
A positive tissue control must be run with every staining procedure performed. This tissue may contain both positive and negative staining cells or tissue components and serve as both the positive and negative control tissue. External Positive control materials should be fresh autopsy/biopsy/surgical specimens fixed, processed and embedded as soon as possible in the same manner as the patient sample (s). Positive tissue controls are indicative of correctly prepared tissues and proper staining methods. The tissues used for the external positive control materials should be selected from the patient specimens with well-characterized low levels of the positive target activity that gives weak positive staining. The low level of positivity for external positive controls is designed to ensure detection of subtle changes in the primary antibody sensitivity from instability or problems with the staining methodology. A tissue with weak positive staining is more suitable for optimal quality control and for detecting minor levels of reagent degradation.
Internal or external negative control tissue may be used depending on the guidelines and policies that govern the organization to which the end user belongs to. The variety of cell types present in many tissue sections offers internal negative control sites, but this should be verified by the user. The components that do not stain should demonstrate the absence of specific staining, and provide an indication of non-specific background staining. If specific staining occurs in the negative tissue control sites, results with the patient specimens must be considered invalid.
Target Information
Collagen IV is a type of collagen protein that is a major component of the basement membrane in various tissues, including the kidneys, lungs, and skin. It forms a network-like structure that provides support and stability to the surrounding tissue. Collagen IV is composed of three alpha chains, which are encoded by the COL4A1, COL4A2, and COL4A3 genes. Mutations in these genes can lead to various genetic disorders, such as Alport syndrome and Goodpasture syndrome, which affect the kidneys and lungs, respectively.
For Research Use Only. Not for use in diagnostic procedures. Not for resale without express authorization.
References (0)
Bioinformatics
Protein Aliases: arresten; COL4A1 NC1 domain; Collagen 4; Collagen alpha-1(IV) chain; Collagen alpha-2(IV) chain; Collagen alpha-3(IV) chain; Collagen alpha-4(IV) chain; Collagen alpha-5(IV) chain; Collagen alpha-6(IV) chain; collagen IV, alpha-1 polypeptide; collagen IV, alpha-3 polypeptide; Collagen IV, alpha-4 polypeptide; collagen IV, alpha-5 polypeptide; collagen IV, alpha-6 polypeptide; collagen of basement membrane, alpha-1 chain; collagen of basement membrane, alpha-4 chain; collagen of basement membrane, alpha-5 chain; collagen of basement membrane, alpha-6; collagen, type IV, alpha 1; collagen, type IV, alpha 3 (Goodpasture antigen); collagen, type IV, alpha 4; collagen, type IV, alpha 5; collagen, type IV, alpha 6; dA149D17.3; dA24A23.1; dJ889N15.4 (Collagen Alpha 6(IV)); Goodpasture antigen; tumstatin; Type IV Collagen
Gene Aliases: ASLN; ATS; BSVD; CA44; CA54; COL4A1; COL4A2; COL4A3; COL4A4; COL4A5; COL4A6; CXDELq22.3; DELXq22.3; DFNX6; ICH; POREN2; RATOR
UniProt ID: (Human) P02462, (Human) P08572, (Human) Q01955, (Human) P53420, (Human) P29400, (Human) Q14031
Entrez Gene ID: (Human) 1282, (Human) 1284, (Human) 1285, (Human) 1286, (Human) 1287, (Human) 1288

Performance Guarantee
If an Invitrogen™ antibody doesn't perform as described on our website or datasheet,we'll replace the product at no cost to you, or provide you with a credit for a future purchase.*
Learn more
We're here to help
Get expert recommendations for common problems or connect directly with an on staff expert for technical assistance related to applications, equipment and general product use.
Contact tech support